Hello my little chef’s! Today we are learning new things in a, hopefully, interesting way! When we first started cooking, we had so many questions no one could/would answer and that’s why we want to help! We’ll try to answer all your problems ranging from intensely frightening French words like charcuterie, to the ultimate “how to put out my kitchen fire” guide! Yea, we will try to help, first with the six basic principles of food science, and then with your kitchen fire!
What are the six basic principles of food science?
There’s a science behind everything and food is no exception. It’s essential for every chef to know a little science to truly be the master of every kitchen! For the most time it is important to know how your food will react in certain situations and cooking conditions. The six basic principles will surely help you with that! They are as follows: Caramelization, Coagulation, Denaturation, Emulsification, Gelatinization and the Maillard reaction.
Why do you need to know the principles of food science?
Cooking is an artform itself, but you can help it out with a little science! The more you learn about cooking, the better you will be at it. Soon you will start to be comfortable with every process happening in your kitchen, on the other hand, if you are experienced in cooking, it will help you to round out your cooking knowledge.
1. What is Caramelization?
The main carbohydrates in foods are sugar and starch! When sugar is heated it will first melt into a syrup and then change color from white – yellow to brown. This browning chemical reaction is called caramelization. The caramelization process involves the removal of water and the breakdown of sugar. Why is this important you ask yourself (my little kitchen Einstein)? Because caramelization brings out whole different cooking aromas (a lot of people like) that are important in a lot of dishes. The main aromas caramelization brings to your palate is butterscotch, sweet rum, nutty, toasty and (if excess caramelization is done) bitter. Imagine your Snickers without caramelization, you can’t, so do we (we won’t even talk about the rum)!
2. Coagulation – “what is done cannot be undone”
If coagulation had a leading role in Sex and the City we guess it would be Samantha, because once coagulation is done, it cannot be undone (ba dum tss). Coagulation is the process of protein transformation from a liquid to a solid form (and why the heck would this be important?). It is important because once the proteins are coagulated, they cannot be returned to their previous (liquid) state. If you ever made Tiramisu, you know what egg yolk coagulation means, but you can find coagulation in every corner of your kitchen (be aware)! Coagulation temperatures range between 38 °C – 70/82 °C (100 °F- 180 °F). The three main types of coagulating proteins are egg, dairy-soy and flour proteins. Coagulation is important in pastry making, desserts, sauce recipes and a lot more!
3. Protein denaturation – changing the structure of proteins
Denaturation is the process of changing the structures of proteins when they are exposed to heat, salt or acid. Contrary to coagulation, some heat induced denaturation is reversible through cooling. That’s why your Sunday roast needs to rest a little, to become nicely moist again, and people say food science isn’t fun!
4. What are emulsions in the culinary arts?
An Emulsion is a mixture of two liquids that normally would not mix together (like oil and vinegar). There are three types of emulsions: permanent (mayonnaise), semi-permanent (Hollandaise sauce) and temporary (vinaigrette salad dressing). Emulsions are an essential part of cooking, either as sauces, dressings, or the ever-loving-favorite mayonnaise.
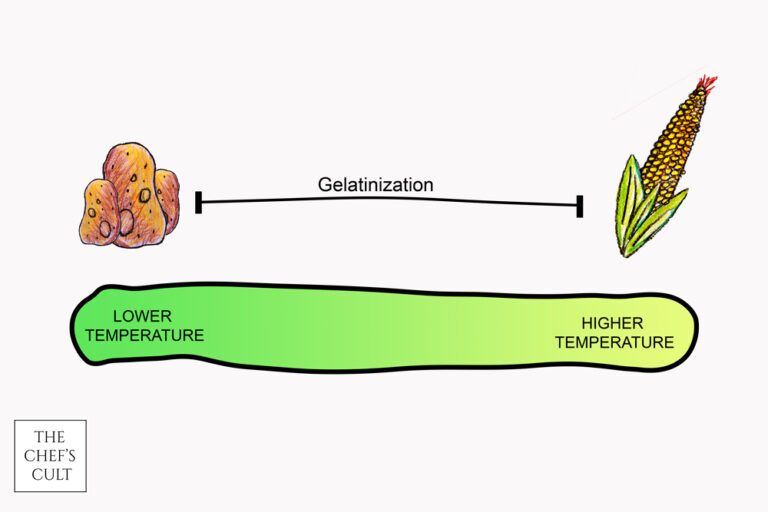
5. Gelatinization – starch thickening process
Starch gelatinization is the process where starch granules swell in presence of heat and water. The result is thickening of the liquid and change in texture. In general, root-based starches (like potato and arrowroot) thicken at lower temperatures, while cereal based starches (like corn and wheat) thicken at higher temperatures. High levels of sugar and acid can inhibit the process, while salt can promote it. Gelatinization is very important in sauces and pasta dishes (when pasta is cooked, the starch swells as it absorbs water, and in the end our pasta softens – and becomes delicious).
6. Maillard reaction – the fancy cooking term everybody uses but a few understand
The Maillard reaction is named after the Frenchmen (who else?) Louis Camille Maillard who first described the reaction between sugars and amino acids at higher temperatures. It’s the chemical reaction between reducing sugars (“simple sugars”) and amino acids which happens, mostly, at higher temperatures and lower surface moisture content.
An important part of the reaction is non-enzymatic food browning which happens mostly at 140-165 °C (280 – 330 °F), and of uttermost importance food flavors change drastically! Maillard reaction is “the Big Bang” of culinary arts, because hundreds unique flavor compounds emerge, depending on the individual food chemistry, cooking time and temperature! Often the reaction is so complex that these new food flavors break down to form even more flavorful compounds.
Imagine the Maillard reaction as the reaction that brings the Bang! to your food! Some examples of Maillard reaction in foods are cookies, biscuits, bread, seared steaks, fried food as dumplings and stir fry, beer, chocolate, coffee, maple syrup, roasted food, grilled food, etc. It’s virtually everywhere around you!
Today you learned the six basic principles of food science, which are an essential part of cooking knowledge and the culinary arts. We congratulate, my little Einsteins, you can now proceed to the master level of cooking, be fun at parties or outsmart your mother-in-law! Wisdom is power, use it for good things, good food in our context, and do not burn down your kitchen! And as always sharing is caring!


